Soak Up this Free Disappearing Water Activity
Use this free activity to whet your students’ appetite for exploring superabsorbent, hydrophilic polymers and their real-world applications.
All grades
Superabsorbent polymers (SAPs) are used for many applications, from hygiene materials to agriculture, food & transportation, construction, medical, etc. Thanks to SAPs, disposable absorbent hygiene products such as diapers and feminine pads have come a long way, baby! The absorption capacity of disposable absorbent products significantly improved after manufacturers began using superabsorbent polymers. Students can discover the science behind SAPs’ ability to absorb up to 1,000 times their own weight in water in the Disappearing Water activity above.
The disappearing water activity demonstrates several scientific concepts, including osmosis, diffusion, absorption, and the structure and properties of matter. Download this activity to whet your students’ appetite for exploring hydrophilic polymers.
How do superabsorbent polymers work?
SAPs are crosslinked networks of flexible polymer chains.1 When dry, the polymer chains are in shrunken coils. When water comes into contact with the polymer chains, they uncoil and expand. Water is trapped tightly within the crosslinked molecular network forming a gel. The main ingredients of SAPs are acrylic acid, sodium hydroxide (or a similar neutralizing agent), and water plus a crosslinker which forms the bridge that links one polymer chain to another.2
Manufacturers convert that mixture into an aqueous gel crushed and dried into superabsorbent granules, ready to be put into handy items like the icepacks in our coolers.
One of the most frequently asked questions of disposable diaper manufacturers is, “What are those crystals and gel I notice in the used diapers?” That question wouldn’t stump the students in your class after your lesson on the mechanism of absorption of SAPs.
What’s the history of superabsorbent polymers?
Before SAPs, manufacturers relied on traditional water-absorbing material such as tissue paper, cotton, sponge, and fluff pulp. So, how did SAPs come into the picture?
In the 1960s, the U.S. Department of Agriculture started to work on materials to improve water conservation in soils. They developed a resin based on the grafting of acrylonitrile polymer onto the backbone of starch molecules.3 They found this new material could absorb more than 400 times its weight and, more importantly, it didn’t release liquid water even under pressure! Psst…if your students do the extension described in the Disappearing Water activity, they’ll discover how to release the liquid by using salt to disrupt the attraction between the water and the polymer chain.
The USDA was not alone in its search for the perfect “super slurper.” Japanese researchers started using starch, carboxymethyl cellulose (CMC), acrylic acid, polyvinyl alcohol (PVA), and isobutylene maleic anhydride (IMA). In the 1970s, SAPs were used commercially for the first time—for disposable hygienic products.3
Your students will be like sponges as they soak up the facts they discover about superabsorbent polymers in the Disappearing Water activity above.
References: 1. Sanyo Chemical: Superabsorbent polymers. 2. Edana: What is SAP – superabsorbent polymers 3. Ma’s Group: History of Super Absorbent Polymer Chemistry
Recommended products:
[StartProductBlock]
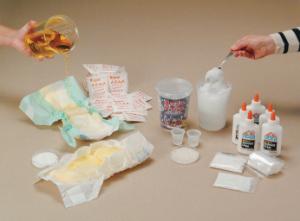
Polymers: An Investigation and Diaper Dissection Kit
Ideal for the students to investigate the many uses and properties of polymers.
[EndProductBlock]
[StartProductBlock]

Ward’s® Essentials Properties of Polymers Lab Kit
Students explore differences between thermoplastic and thermoset polymers and the properties of various crosslinked, superabsorbent polymers.
[EndProductBlock]
[StartProductBlock]

Ward’s® Chemistry Instant Snow Demonstration
Students observe the superabsorbent property of a chemical polymer.
[EndProductBlock]
[StartProductBlock]
Potassium Polyacrylate
Synonyms: Acrylamide, Potassium Acrylate Copolymer, crosslinked.
[EndProductBlock]
